Abstract
Introduction:
Cancer-associated thrombosis (CAT) is a significant cause of morbidity and mortality in cancer patients. Several mechanisms underlying the development of thrombosis in cancer patients have been described. An underappreciated role for the contact activation pathway in pathologic thrombosis has become apparent in recent years, though its role in thrombosis induced by malignant disease has not been defined. It has been suggested that one mechanism for activation of the contact pathway may involve activation of factor XII (FXII) to FXIIa by extracellular vesicles (EV). To further explore this mechanism, we have isolated EV from several cancer cell lines and assessed their ability to cause FXII activation in normal human plasma.
Methods:
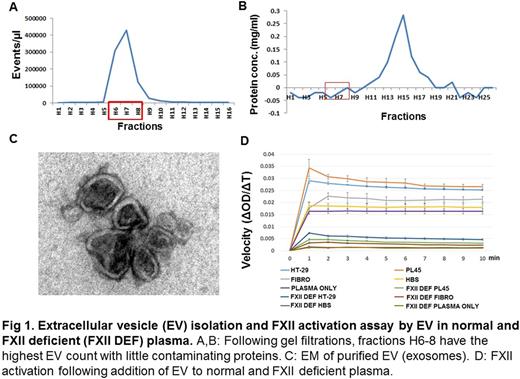
EV were isolated using a multistep ultrafiltration and ultracentrifugation method from HT29 (colorectal), U87 (glioblastoma), and PL45 (pancreatic) cell lines, as well as from primary cultures of human umbilical vein-derived fibroblasts, used as a control. EV were quantified by measuring protein concentration, and equal amounts of EV, as judged by protein content, were added to citrated human plasma (Fig. 1 A, B, C). FXIIa generation was quantified colorimetrically using the substrate H-D-Prolyl-L-phenylalanyl-Larginine-p-nitroaniline dihydrochloride (S-2302). Dextran sulfate was used as a positive control to induce FXII activation. Briefly, EV (one microgram by protein content) and S-2302 were added simultaneously to 40 microliters of normal human plasma, after which A405 was recorded.
Results:
We observed that all EV tested caused activation of FXII. However, the initial rate of FXII activation, as determined by comparison of the A405 at 1 minute, was between 80-300% higher in the presence of EV derived from cancer cell lines compared to that observed in the presence of fibroblast EV. Moreover, consistent with clinical observations suggesting that pancreatic cancer is associated with a particularly high incidence of thrombosis, we observed that EV from the PL45 cell line was the most potent in inducing FXII activation, in which the initial rate is about 120% or 300% higher than that of other cancer cell lines or fibroblast, respectively (Fig. 1D). To assess the specificity of the FXII substrate used in these studies, we performed parallel experiments using FXII deficient plasma, observing essentially no substrate conversion over the initial one minute assay period.
Conclusion:
These studies demonstrate that EV derived from both primary, non-transformed cells, which have generally been omitted from prior studies assessing the prothrombotic activity of EV, as well as cancer cell lines directly activate FXII in human plasma, though EV from cancer cell lines do so more potently and in accordance with the predicted thrombosis risk associated with the parental cancer cell type. Results with FXII-deficient plasma demonstrate that substrate activation is due to FXIIa rather than kallikrein. Current studies are focused on measuring the ability of EV isolated from patients with and without malignancy to activate FXII, and on defining the mechanisms of FXII activation, particularly the role of polyphosphate. Inhibition of contact pathway activation may be a new approach to treatment and prevention of CAT, particularly since patients with cancer are at higher risk for bleeding than normal individuals while on anticoagulation.
Khorana: Sanofi: Consultancy, Honoraria; Halozyme: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Janssen Scientific Affairs, LLC: Consultancy, Honoraria, Research Funding; Leo: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal